HMO study paves the way for developing next-generation infant formula

Image: Iuliia Bondarenko / Pixabay
HMOs — important groups of carbohydrates in human breast milk — can, in some cases, be secreted in very high concentrations, and still be safe and well-tolerated by infants.
This is the conclusion of a new study by global bioscience leader Chr. Hansen, published in Food and Chemical Toxicology. The systematic review compiles data from several dozens of observational peer-reviewed studies for the five most prevalent HMOs in breast milk. It presents the largest data set analysed to date and provides state-of-the-art information to support the appropriate and safe levels of HMO supplementation in infant formula.
Whereas studies have so far focused on the quantitation of HMOs in human milk, this review determines the natural concentrations of HMOs. The concentration levels vary depending on the mother’s health and genetics, environmental and geographical factors, gastational age (pregnancy progression) and lactation stage. The objective of the new study was to provide a clearer perspective on natural HMO concentrations and distribution in breast milk, as this is important to develop next-generation infant formula products with an HMO composition that is closer to breast milk.
Closer to nature and respecting natural variations
“Breastfeeding is the best way to ensure infant health and recommended by WHO. At Chr. Hansen, we further aim to support the healthy development of infants that cannot be breastfed by providing HMOs as an ingredient and blend for infant formula. We are excited to publish this study of the five most prevalent HMOs in breast milk, which are all included in Chr. Hansen’s 5 HMO Mix in concentrations closer to nature and respecting the natural variations,” says Jesper Sig Mathiasen, senior vice president, Chr. Hansen HMO.
“The study presents important statistical data to help support the level of appropriate HMO supplementation in infant formula and confirms the safety of intake at concentrations higher than average. We see it as yet another testimony to our HMO offering,” he notes.
Conclusions of the study
Out of over 150 HMOs identified in human breastmilk, the five most prevalent and best studied HMOs are 2′-fucosyllactose (2′-FL), 3-fucosyllactose (3-FL), Lacto-N-tetraose (LNT), 3′-sialyllactose (3′-SL), and 6′-sialyllactose (6′-SL).
Results show a wide distribution of HMO concentrations in breast milk, ranging up to as much as 10 g/L for the most prevalent one, 2’-fucosyllactose (2’-FL). See figure 1 below.
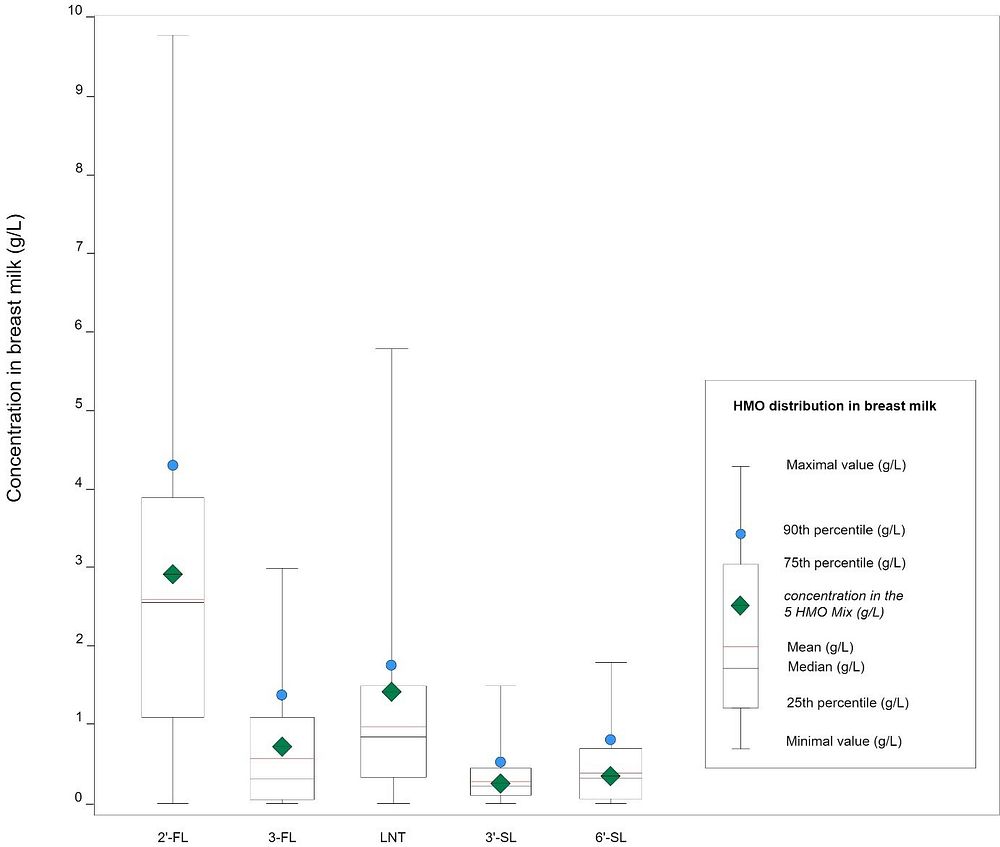 |
Fig. 1: HMO distribution in breast milk (adapted from Parschat et al., 2022)
The safety, tolerability, and health benefits of Chr. Hansen’s 5 HMO-mix have already been demonstrated in previous scientific studies (1). Infants fed infant formula containing the 5 HMO-mix demonstrated similar digestive parameters and stooling patterns as breastfed infants.
Developed in 2019, Chr. Hansen’s 5 HMO Mix is already commercially available in North America, with approvals pending in Europe, Asia, and Latin America.
